Authorized for Emergency Use by the FDA
Provides results within just 10 minutes
-
Intended for use with whole blood, serum or plasma samples
-
Detects IgM that may appear in blood within 3-5 days following incubation and IgG that appears as soon as 1-2 weeks
-
Can be stored at room temperature or in a refrigerator
-
Shelf life of up to 24 months from the date of manufacture
COVID-19 Rapid Test Kit “Coronavirus” FDA Emergency Use Authorized IgG/IgM
SARS-CoV-2 IgG/IgM (Whole Blood/Serum/Plasma) Rapid Test Device
applies lateral flow technology that can be used for the detection of
both anti-SARS-CoV-2 IgM and IgG antibodies. Our SARS-CoV-2 rapid test
analyzes the patient’s response to SARS-CoV-2 after the infection
begins and gives a qualitative result between 2-10 minutes. Primarily,
antibodies can be found up to 21 days after infection. It can be used
for the rapid detection of carriers of the virus that are symptomatic
or asymptomatic.
The COVID-19 ‘Coronavirus’ rapid test kit is conducted by using a small sample of whole blood, serum or plasma. By placing the suggested amount of sample on the cassette, then adding two drops of the buffer labeled “B.” Results for this COVID-19 test should appear within10 minutes (actual minutes can vary). Any results after 10 minutes should be deemed invalid. For more information, watch the video for this instant test kit.
For questions or pricing information please call us at (866) 788-2855
-
This test has been authorized by FDA under an EUA for use by authorized laboratories
This test has not been FDA cleared or approved
-
This test has been authorized only for the presence of IgM and IgG antibodies against SARS-CoV-2, not for any other viruses or pathogens.
-
This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
Suitable for the qualitative detection of Coronavirus (SARS-CoV-2 / COVID-19) (Suggestion below)
Qualitative test for the detection and differentiation of IgM and IgG antibodies against SARS-CoV-2 in venous whole blood, plasma (Li+-heparin, K2-EDTA and sodium citrate), and serum. The product is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies confers protective immunity.
Detection Window IgM:
Symptomatic 3-5 days
Asymptomatic 7 days
Sample: This instant COVID-19 test kit can work with whole blood, plasma, and serum samples.
Shelf Life Expectancy: 24 months from manufacture date (date to be determined at the time of sale, please ask our associate for the exact manufacturer date)
Storage: The kit should be stored at room temperature or refrigerated (2-30°C).
Call The DrugTestsinBulk.com Customer Service Team to Request More Information About Our COVID-19 instant test kit available to medical professionals.
*Please note, This Coronavirus instant test kit has been authorized only for the presence of IgM and IgG antibodies against SARS-Cov-2, not for any other viruses or pathogens. Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
*Please inquire with Drug Tests In Bulk to receive more detailed information on clinical evaluations and test stability reports.
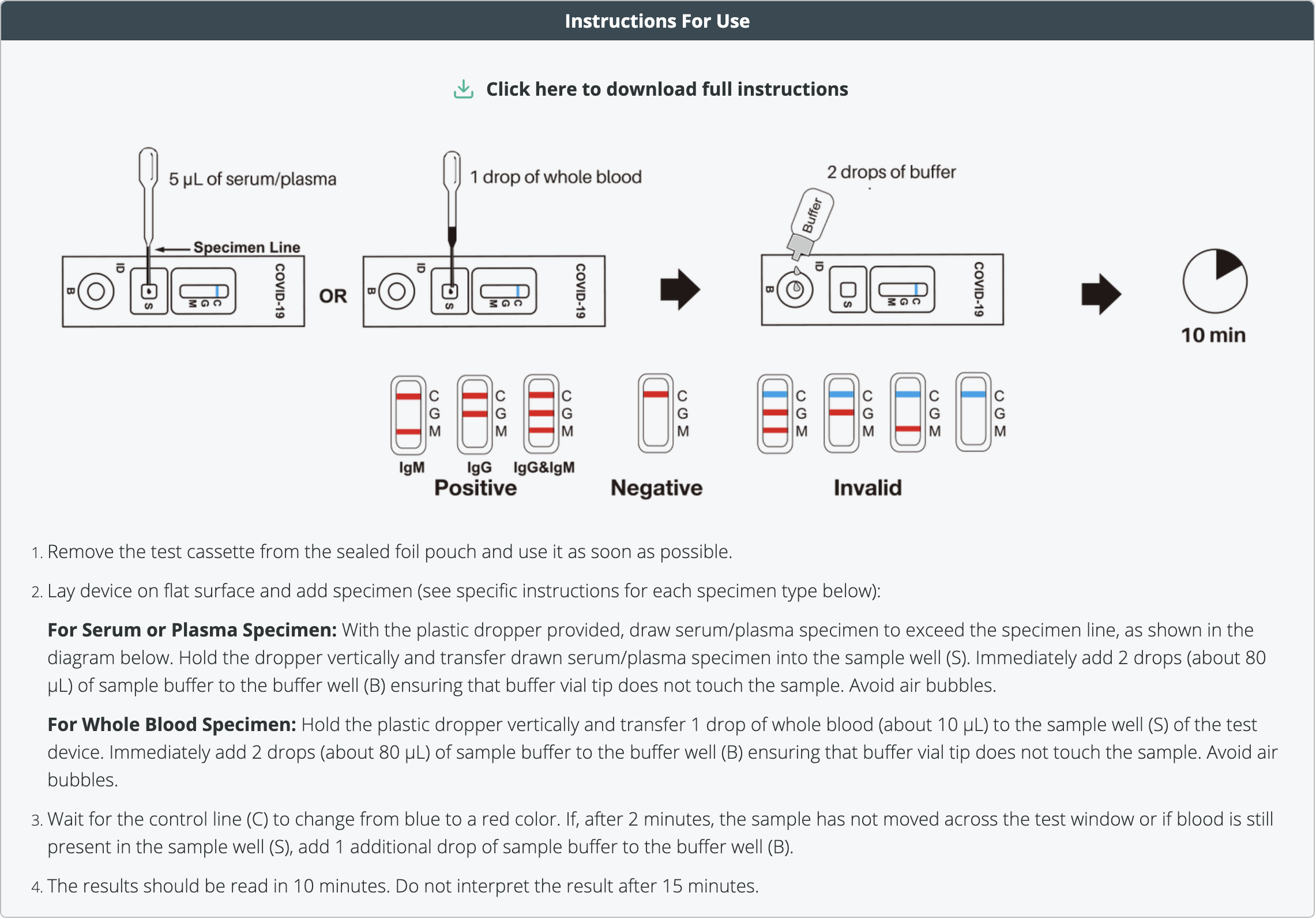
First, remove the COVID-19 instant test cassette from the manufactured sealed foiled pouch and use it as soon as possible once opened. For best result obtain the sample within one hour of opening the test.
Then place the ‘coronavirus’ testing device on a clean & level surface.
(For Whole Blood Specimen): Hold the 5 μL mini plastic dropper vertically and transfer 1 drop of whole blood (about 10 μL) to the specimen well(S) of the test device. Then add 2 drops (about 80 μL) of provided sample buffer to the buffer well (B) immediately. Avoid air bubbles when performing these steps.
-
(For Serum or Plasma Specimens): With a 5 μL mini plastic dropper provided, draw serum/plasma specimen to exceed the specimen line and then transfer drawn serum/plasma specimen into the sample well (S). Then add 2 drops (about 80 μL) of sample buffer to the buffer well (B) immediately. Avoid air bubbles. Wait for the colored line(s) to appear. The result of our COVID-19 test should be read within 10 minutes. Positive results may be visible as soon as 2 minutes. Any results after 10 minutes should be deemed invalid.
Each box contains 25 individually wrapped tests. There is a 1 box minimum order for each sale.
Our COVID-19 IgG/IgM Rapid Test Cassette can be used with whole blood, serum, or plasma samples.
Results will appear within ten minutes.
We carry a large inventory of COVID-19 IgG/IgM tests. It is best though to speak with your sales associate to make sure we have the necessary inventory to accommodate your order.
Our COVID-19 test kits include 25 individually wrapped tests along with 1 pipette. 1 bottle of buffering agent. Instructions for use.
Yes. Our product was granted an Emergency Use Authorization (EUA) from the FDA on May 29, 2020.
Following the incubation period, IgM may appear in blood within 3-5 days. IgG will appear as soon as 1-2 weeks.
No. This test is for professional medical use only.
No. This test is not CLIA Waived.
The test uses lateral flow technology that is used for the qualitative, differential detection of anti-SARS-CoV-2 IgG and IgM antibodies. This test is intended to screen patients, symptomatic or asymptomatic, for the COVID-19 in Human whole blood, serum or plasma.
Yes. If you are positive for either IgG, IgM or both, you will need a confirmation swab test.
Please see manufacturer’s instructions in the product insert. Simple explanation if you are using whole blood: Our Rapid Cassette Test requires one drop of blood along with 2 drops of a buffer. Interpret results within ten minutes.
The U.S. FDA has made this test available under an emergency access mechanism called an Emergency Use Authorization (EUA). The EUA is supported by the Secretary of Health and Human Service’s (HHS’s) declaration that circumstances exist to justify the emergency use of in vitro diagnostics (IVDs) for the detection and/or diagnosis of the virus that causes COVID-19.
An IVD under an EMERGENCY USE AUTHORIZATION (EUA) has not undergone similar review as an FDA-approved or cleared in vitro diagnostics device. The U.S. Food & Drug Administration has the authority to issue an EUA when certain criteria are met, which includes that there are no adequate, approved, available alternatives, and based on the completeness of scientific evidence available, it is acceptable to believe that this in vitro diagnostics device (IVD) may be adequate.
The EUA for the test you received is in place for the duration of the COVID-19 declaration justifying emergency use of IVDs, unless cancelled or revoked (the test may no longer be used).
The Covid-19 IgG/IgM Rapid Test Cassette can be utilized to screen patients associated with having been influenced by the novel coronavirus. Results of this test should not be the sole basis for diagnosis. Results from Antibody tests should be used in association with other testing methods such as a PCR test.
- Groups – Reasonable priced, quick turn-around testing of symptomatic patients can lower the burden on healthcare facilities, hospitals, and clinics.
- Patients – Rapid tests used on patients showing a Presumptive antibody results for patients with symptoms, allow medical facilities to reduce Healthcare Provider/Patient time, further reducing the chance of contamination in a healthcare setting.
- Immunity – The Covid-19 IgG/IgM Rapid Test Cassette can be used on Recovering or recovered patients in conjunction with approved nucleic acid tests to show recovery and the existence of IgG antibodies fighting the virus in the blood stream.
- Time – The Covid-19 IgG/IgM Rapid Test Cassette can provide presumptive qualitative results onsite in a matter of minutes. Providing healthcare workers with more information at important moments in the patient’s care.
Please use this LINK to download our Healthcare Provider Fact Sheet.
Please use this LINK to download our Healthcare Recipients Fact Sheet.
For product in stock, your order can ship the same if next day. All shipping available such as ground, overnight, 2 day and 3-day shipping.
Many people who have been positive confirmed for COVID-19 have shown symptoms of fever and/or acute respiratory illness (such as: cough, fever, and difficulty breathing). The current information available to characterize the spectrum of clinical illness associated with COVID-19 suggests that symptoms include cough, shortness of breath or dyspnea, fever, chills, myalgias, headache, sore throat or loss of taste or and or smell. What we know about the virus that causes COVID-19, signs and symptoms may show up any time from 2 to 14 days after being exposed to the virus. Based on preliminary data, the median incubation timeframe is approximately 5 days, but has been known range 2-14 days.
Health officials have found cases of COVID-19 infection throughout the world, including the United States, which clearly poses risks to the public’s health. Please check the CDC webpage for the most recent information.
The Covid-19 IgG/IgM Rapid Test Cassette should not be used to diagnose or exclude acute infection and should not be used as the sole basis for treatment or patient management decisions. Direct testing for SARS-CoV-2 should be performed if acute infection is suspected.
Use appropriate personal protective equipment when collecting and handling specimens from individuals suspected of having COVID-19 as outlined in the CDC Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with Coronavirus Disease 2019 (COVID-19). For additional information, refer to CDC Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons Under Investigation (PUIs) for Coronavirus Disease 2019 (COVID-19).
- Why Are Rapid COVID-19 (Coronavirus) Tests Important?
- COVID-19 Coronavirus: What It Is, How It Spreads, What We Can Do to Help
- What are N95 Face Masks and Where Do I Order N95 Face Masks From?
- COVID-19 Rapid Detection Kits Now Available!
- Rolling updates on coronavirus disease (COVID-19)
- Coronavirus disease (COVID-19) pandemic
- CDC Information

